Developing pandemic-busting vaccines in 100 days
The world can face down the next Disease X with a new vaccine in just 100 days. Hereâs howâŚ
COVID-19, as terrible as it has been, is far from the worst case imaginable. A hundred years ago, the world suffered an influenza pandemic caused by a virus that was four to five times more lethal than COVID-19, and in this century alone we have already experienced outbreaks caused by coronaviruses (SARS and MERS) that are respectively approximately 20 and 70 times as lethal as SARS-CoV-2. The only thing that prevented a catastrophe of unfathomable proportions was that they lacked COVIDâs transmissibility.
Vaccines are at the heart of how modern societies counter infectious disease threats. They are our most potent tool against pandemic risks and will be critical to any future response. The faster an effective vaccine is developed and deployed, the faster an incipient pandemic can be contained and controlled.
łÉČËVRĘÓĆľâs aspiration is for the world to be able to respond to the next Disease X with a new vaccine in just 100 days. Thatâs a little over 3 months to defuse the threat of a pathogen with the potential to cause a pandemic. Coupled with improved surveillance providing earlier detection and warning, and with swift and effective use of non-pharmaceutical interventions such as testing, contact tracing and social distancing to suppress disease transmission, delivering a vaccine in 100 days would give the world a fighting chance to extinguish the existential threat of a future pandemic virus.
łÉČËVRĘÓĆľ likes to call this its âmoonshotâ missionâbecause the goal, while ambitious, is technologically within reach.
To some, the goal sounds impossibly far-fetched â until you break the problems that need to be solved down into manageable chunks and think each one through.
The story of COVID-19 helps us do thatâbecause planning for what is possible in the future is grounded in what weâve already shown is possible right now.
Whatâs possible now
If we look across the global portfolio of COVID-19 vaccinesâlife-saving products that were created from scratch, then manufactured, tested, trialled and brought to bear against a completely new diseaseâmany fast-paced possibilities are clear:
- Itâs possible to design a vaccine candidate within 2 days of the genetic sequence of a new virus being published. We know, because thatâs what the NIAID Vaccine Research Center did.
- Itâs possible to move into first human trials in 66 days from the release of the genetic sequence. We know, because thatâs what Moderna did.
- Itâs possible to publish the first safety data 63 days after a Phase 1 clinical trial starts. We know, because thatâs what Moderna did.
- Itâs possible to go from first human clinical trials to vaccine registration in about 7 months. We know, because thatâs what Pfizer / BioNTech did.
- And itâs possible to get emergency use approval within 1 day of filing required data with regulators. We know, because thatâs what Chinaâs CanSino did.
Had these kinds of milestones been achieved for a single COVID-19 vaccine candidate, then a safe and effective vaccine could in theory have been available for use, based on Phase 3 results, more than two months sooner than in this pandemic. That would have shortened the time from âlab to jabâ, or from the publication of a genetic sequence to getting a new vaccine into arms, to less than 9 months. And thatâs without any change to our current regulatory paradigm.
Prepare to prevent
Before the SARS-CoV-2 virus emerged, the previous vaccine-development record was for a live attenuated mumps vaccine, which took a little less than 5 years, or almost 1,800 days, from virus isolation in March 1963 to licensure in January 1968. By comparison, the 326 days it took from the SARS-CoV-2 virus being identified to bringing a COVID-19 vaccine into emergency use represented a quantum leap. Adding to that remarkable progress, is the identifiable evidence that if we look at the best-in-class timelines achieved at various stages, we could, today, shorten the overall timelines by at least another two months.
With tightening and shortening at each stage, and by rethinking how we establish safety and efficacy for emergency countermeasures, cutting that timeline to 100 days should be possible. In a recent interview about the 100-day vaccine plan, Eric Lander, a geneticist, microbiologist, mathematician, and science advisor to President Joe Biden, put it like this: âIt makes you gulp for a second, but itâs totally feasible.â
Itâs all about being properly prepared.
And being properly prepared for the next Disease X means bringing science, leadership and humanity together in a way not previously seen.
Scientifically, itâs about making advances across the whole life cycle of pandemic preparednessâfrom the moment a new pathogen is identified, through the rapid prototyping of a vaccine candidate, through the testing and authorization of that vaccine to get it into to the arms of people at risk.
Economically, itâs about being prepared to make large, bold investments to speed up the building of our defences against emerging threats, even when many of those investments wonât pay off.
Politically itâs about recognizing the scientific opportunity, accepting the moral obligation to try, and crucially, making it work for everyone, not just those fortunate enough to be born in wealthy countries.
In detail, shrinking vaccine development timelines to 100 days involves a series of incremental and shortened steps like the ones we took during the COVID-19 pandemic, combined with some substantial but carefully calculated leaps.
For starters, the world now really has got to improve disease surveillance globally. After multiple warning signs in the form of emerging diseasesâSARS-CoV-1, MERS, Ebola, H1N1âthe need for distributed early-warning systems that identify and track emerging epidemic threats is undeniable.
GISAIDâa global science platform for sharing virological, clinical and epidemiological data about flu âstepped up during the pandemic and began curating SARS-CoV-2 genetic data submitted from around the world, providing critical genomic surveillance for new variants in the process. The new WHO Hub for Pandemic and Epidemic Intelligence in Berlin will pool and share intelligence from global viral surveillance to alert authorities to emerging epidemic threats.
This souped-up disease surveillanceâwhich embeds scanning, searching, and alerting of potential outbreaks as an everyday constant of lifeâis enhanced by us knowing with far more certainty what weâre looking out for. For instance, the WHO has identified a list of pathogens which present known epidemic threats â and may be related to the next COVID-19, just as COVID-19 is genetically related to two diseases (SARS and MERS) weâd already encountered.
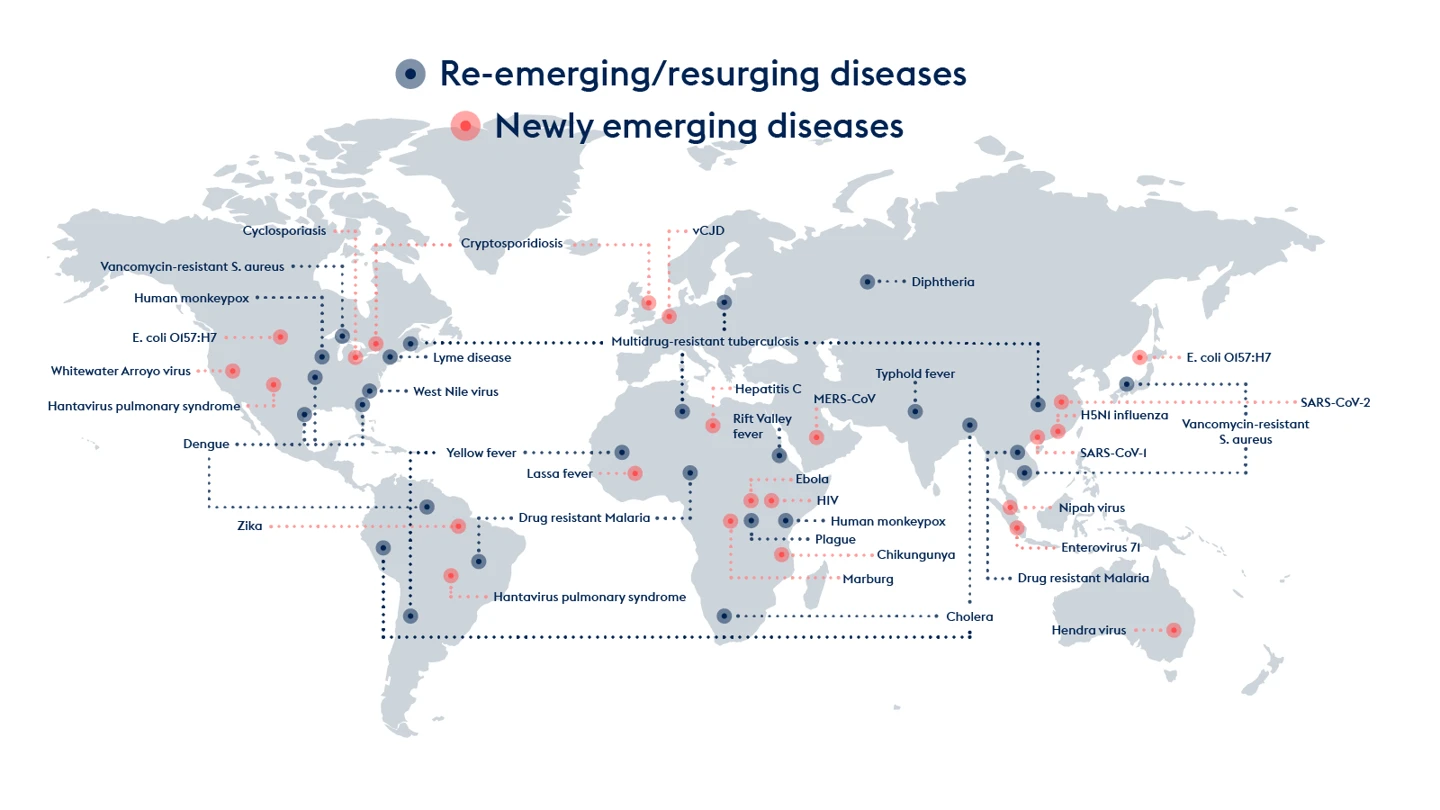
Pathogens continually emerge and re-emerge: from human monkeypox and drug-resistant malaria to typhoid and plague, the deadly Nipah and MERS viruses, the frightening strains of bird flu, and the mosquito-borne Zika and Chikungunya viruses.
Any cousin of these could be the next Disease X. Not all new diseases have pandemic potential, but the next one that does could be as bad as COVID-19, if not worse.
Knowing the (unknown) enemy
In preparing for Disease X, itâs important to be clear about the knowns and the unknowns: The X in âDisease Xâ stands for everything we donât know. Itâs a new disease, about which we will know very little when it first emerges: it may or may not be deadly, highly contagious and a threat to our way of life. We also donât know when, and donât know how, it will come across the viral frontier and infect people.
łÉČËVRĘÓĆľ know is that the next Disease X is coming and that we have to be ready.
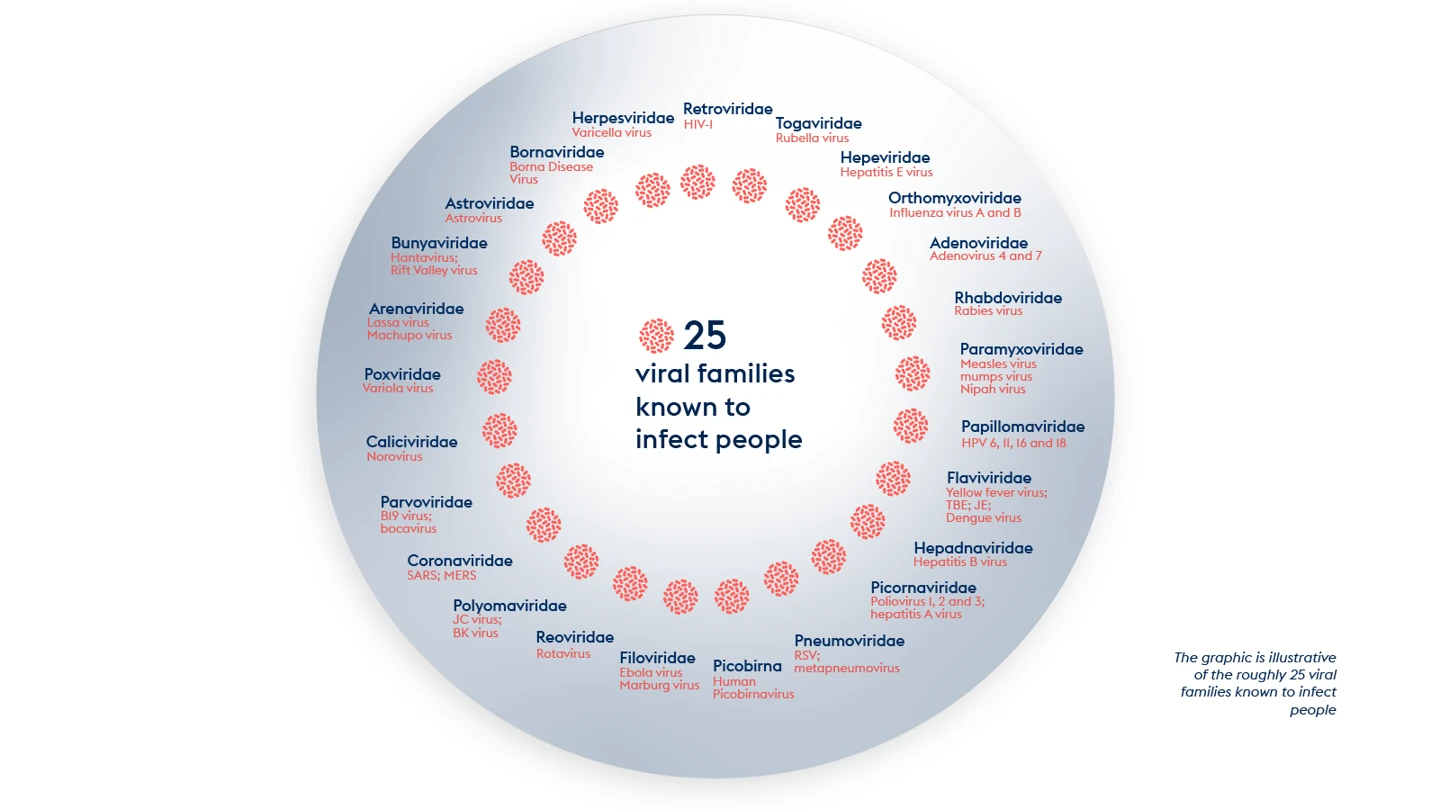
There are around 260 viruses known today to be able to infect humans. Those 260 or so viruses belong to roughly 25 viral families.
These viral families include:
The paramyxovirus family, which counts the highly contagious ancient diseases measles and mumps and the more recently identified and much more lethal Nipah and Hendra among its members.
The coronavirus family, which includes both mild but highly contagious common cold viruses as well as COVID-19 and the even more deadly MERS and SARS.
The arenavirus family, the first identified member of which was isolated in the 1930s, and which includes a number of Old World and New World viral haemorrhagic fevers.
The filovirus family, which gives us Ebola and the equally frightening Marburg.
The orthomyxovirus family, the best-known members of which are the influenza viruses that have caused at least four pandemics since 1918.
And the flavivirus family, many of which, not least Yellow Fever, Dengue, and Zika, are transmitted by mosquitoes or other insects and now have spread globally.
The full list of âneighbour from hellâ viral families is here:
Solving the science in advance
Realistically, we canât create individual new vaccines against 300 or more potential or developing threats let alone the 1.6 million or so more viral species that may exist in mammal or bird hosts and have yet to be discovered.
But we can develop vaccines against prototypes of these threats. In other words, we can focus our efforts on pathogens that exemplify some or all of the worst traits a particular viral family.
By solving the problems of vaccine development in advance for a particular pathogen, we prepare to develop vaccines against other members of its family much more rapidly than if we had to start from scratch.
Itâs true that the vaccines against the previously unknown SARS CoV-2 virus were developed and deployed at unprecedented speed.
But that achievement didnât come from nowhere, and that innovation didnât come out of the blue. Just like other recording-breaking human achievementsâbe they Usain Boltâs 9.58 second 100m sprint, Dick Rutanâs and Jeana Yeagerâs non-stop flight around the globe, or the Apollo 11 moon landingâthey were built on years of hard work: learning, training, experimenting, and testing.
The story of COVID-19 vaccine success shows us how a prototypic-vaccine approach works.
The years of diligent work included painstaking research into vaccines for two closely related coronaviruses, SARS-CoV-1 and MERS-CoV.
Vaccines protect by helping the immune system sense and target invading pathogens. Vaccines typically do this by presenting or fragment of the pathogen â a so-called âimmunogenâ â to the immune system to elicit a response before the patient is infected.
For MERS and SARS and SARS-CoV-2, the critical immunogen is the spike protein on the surface of the virus. The spike protein allows each virus to gain entry into cells but is also the major target of the immune system. Through their work on MERS and SARS vaccines, scientists had learned how to elicit a strong immune response against the coronavirus spike protein. These techniques were critical advance knowledge when it came to designing vaccines against the new coronavirus threat of SARS-CoV-2.
Also important in the story of COVID-19 vaccine development were the extraordinary leaps forward in vaccine technology that came to fruition at precisely the right moment.
As part of their work in those years, scientists had honed and developed so-called rapid-response platforms to make âplug-and-playâ vaccines. Some used mRNA technology, and others, like the University of Oxfordâs ChAdOx, used viral vectors. This meant that when Disease X came and was identified and named as SARS CoV-2âvaccine makers could plug in the genetic code for a part of the novel coronavirus that would trigger an immune response.
These head starts, and the speed with which vaccines were developed as a consequence, have effectively served as âproof of conceptâ for a prototypic vaccine approach. COVID-19 showed us how it worked as way of moving fast against a totally new virus, and how it can also work in future against other yet-to-emerge pathogens.
łÉČËVRĘÓĆľâs plan, depending on the complexity and diversity of viruses within a given family, is to create a range of vaccine candidates (perhaps up to 15 in the most complex families) for each family then to select a handful as prototypes to take through rigorous testing including safety and dosing trials in people.
In this way, when a newly emerging virus comes across the frontier â and that is most certainly a âwhenâ and not an âifâ â weâll have banked a vast amount of data on safety and immunogenicity of both the plug-and play platform technology, and the antigens of viruses closely related, if not exactly the same, as the new Disease X.
Ultimately, the gains made through global surveillance and early detection and alerting, coupled with earlier suppression efforts, will mean that on our Day Zeroâthe start of the 100-day countdown to develop a vaccine to halt an outbreakâcase numbers might still be relatively small. And with a vast library of knowledge built up, inserting a genetic sequence for a new and threatening Disease X means weâll already be months ahead in constraining not just the outbreak and the novel virus, but crucially, its pandemic potential.
Alongside the pre-pandemic scientific problem solving, neutralising a future Disease Xâs pandemic threat will also require the world to agree some calculated shift on clinical trials and regulation. With the head-start knowledge weâll l have banked in the library of prototype vaccines we can get a head start on designing the trial protocols, getting much of the administrative, and intellectual work done in âpeace timeâ.
łÉČËVRĘÓĆľ is already working on establishing a global network of clinical trial sites and labs that will need to work with clearly defined ârules of the roadâ in specific pandemic threat scenarios. This will also mean authorities agreeing global clinical trial protocols for use in emergency situations and establishing pre-enrolled ready-to-go cohorts of people in different countries, different regions and across different age-groups and ethnicities. This will enable clinical trials to start almost immediately, and mean they can run in parallel with rapid regulatory reviews to assess the benefits of a vaccine balanced against risk of infection and death in a novel outbreak.
If thereâs one thing we found out during the COVID-19 crisis, itâs that pandemics are devastatingly expensive. By the end of 2025, it is forecast that COVID-19 will have cost the world $28 trillion over 5 years. Thatâs $28,000,000,000,000. And thatâs just the economic cost. We will never be able to quantify the human cost of this pandemic, but its effects will no doubt reverberate for generations.
For sure, being properly prepared doesnât come cheap. Creating the vaccine library could mean developing as many as 100 prototype vaccines to ensure weâve got something in the knowledge bank to help us cover almost any threat. Yes, thatâs a lot, but itâs finite. Itâs also eminently achievable if governments and industry work together. For its part, łÉČËVRĘÓĆľ has a US$3.5 billion pandemic-busting plan that, over the next 5 years, will kickstart and coordinate this work. Compared with the trillions lost to COVID-19, at $3.5 billion this plan is not only value for money, itâs exactly what the world needs to ensure our children never again face the hardship and loss weâve had to endure from COVID-19.
We must invest today, to save tomorrow.
