łÉČËVRĘÓĆľ partner IAVI announces first vaccinations at Liberia site in Phase I clinical trial of Lassa Fever vaccine candidate
Share
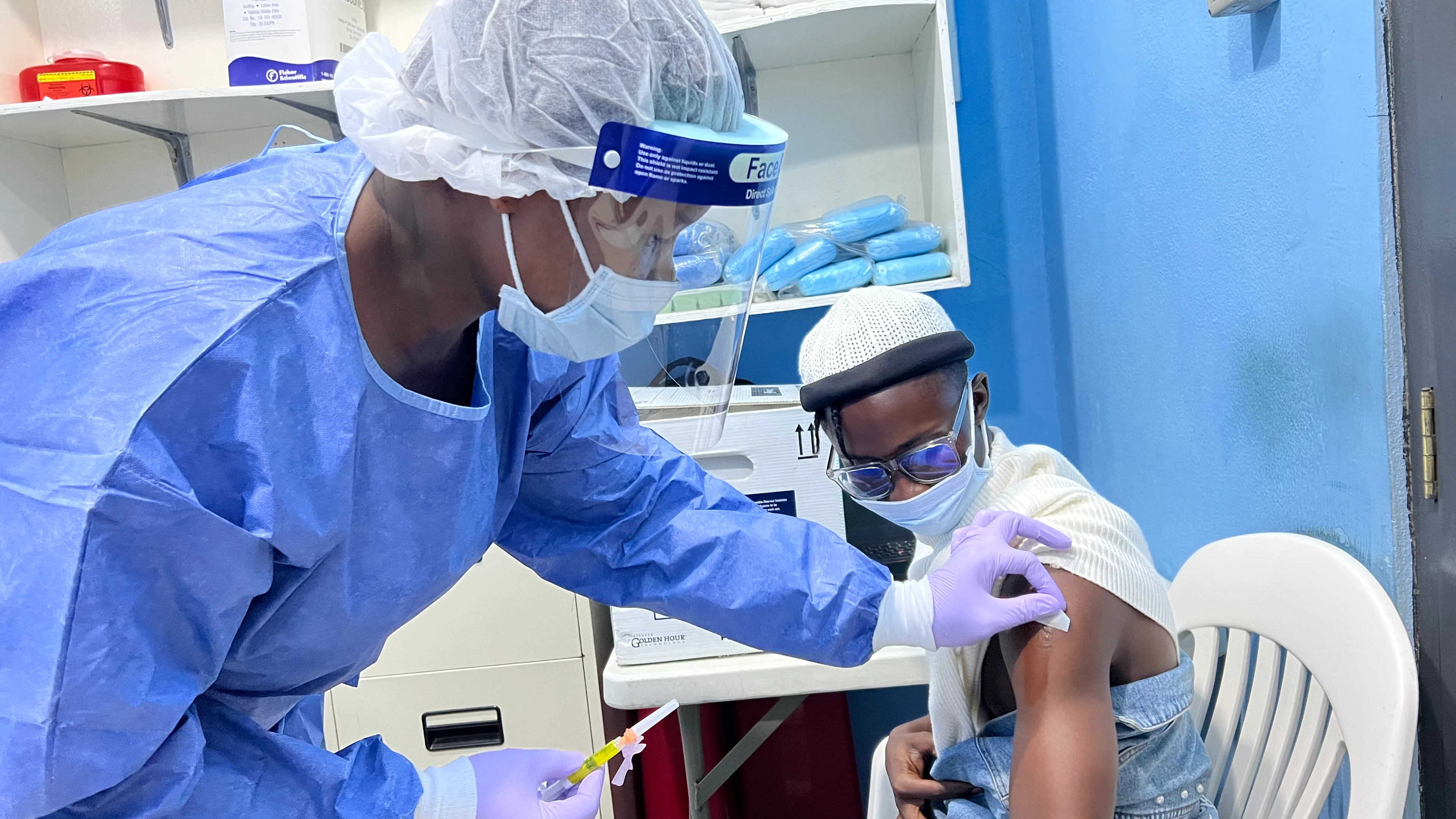
NEW YORK â AUG. 31, 2022 â IAVI, a nonprofit scientific research organization, announces that volunteers at the PREVAIL clinical trial site at Redemption Hospital (RH)* in Monrovia, Liberia, have been vaccinated with IAVI's novel vaccine candidate against Lassa fever virus (LASV) in a Phase I clinical trial, named IAVI C102, supported by the Coalition for Epidemic Preparedness Innovations (łÉČËVRĘÓĆľ).
LASV causes significant annual outbreaks of an acute viral illness called , which is endemic, or consistently present, in many parts of West Africa. Outbreaks in Guinea, Liberia, Nigeria, Sierra Leone, South Africa, and Togo have resulted in nearly 6,000 suspected cases and more than 180 deaths since early 2022, according to by the World Health Organization (WHO). The WHO also three recently confirmed cases of and one death from Lassa fever in the U.K. No vaccine for LASV is currently available. An estimated 300,000 to 500,000 Lassa fever cases are reported annually, resulting in approximately 5,000 deaths. However, the true disease burden is currently unknown, and efforts are ongoing to provide a more accurate estimate of disease incidence.
The IAVI-sponsored trial is part of the , launched in 2018, providing up to US$61.7 million to support IAVI and a global consortium of partners to advance IAVI's LASV vaccine candidate through Phase I and II clinical trials. łÉČËVRĘÓĆľ's ultimate goal, as part of its plan to minimize or even eliminate the risk of future epidemic and pandemic threats, is to produce a licensed Lassa vaccine for routine immunization.
Clinical evaluation of IAVI's LASV vaccine candidate is also supported by the Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL**), a Liberia-U.S. clinical research collaboration established in 2014 by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Ministry of Health in Liberia. In 2015, PREVAIL participated in a clinical trial () of Merck's now-licensed Ebola Zaire vaccine ERVEBOÂŽ, which uses the same recombinant vesicular stomatitis virus (rVSV) vector backbone as the candidates in IAVI's (EIDs) vaccine development portfolio, including the IAVI LASV vaccine candidate being evaluated in C102. Read more: .
Continued Lassa fever outbreaks underscore the need to realize rapid vaccine development and plan for widespread access before and during disease outbreaks.
We believe that IAVI's rVSV technology offers a promising and validated new platform for development of vaccines that target outbreak-associated pathogens like Lassa fever virus.
The experienced, broader network of collaborators in the IAVI C102 trial bring expertise in both rVSV vaccine development and in conducting clinical trials in endemic settings. Together, we'll glean important safety, tolerability, and immunogenicity data while strengthening the individuals, institutions, and technology needed to develop urgently needed Lassa fever vaccines.
The prospect of developing a vaccine that will prevent Lassa fever is a great step forward. A Lassa fever vaccine will greatly reduce the disease burden by flattening the epidemic curve of seasonal outbreaks, offering hope and relief to local populations, especially rural communities who continue to suffer the brunt of this disease.
is designed to evaluate the vaccine candidate's safety, tolerability, and ability to elicit an immune response against Lassa fever among approximately 100 healthy adults in the U.S. and Liberia. Vaccinations have already begun at the Brigham and Women's Hospital, East-West Medical Research Institute, and George Washington University School of Medicine and Health Sciences in the U.S. Each participant will receive at least one dose of either the study product or placebo and will be monitored for safety for up to one year following vaccination.
IAVI and łÉČËVRĘÓĆľ are united in their commitment to global equitable access to vaccines, with IAVI's LASV vaccine candidate to be accessible to all populations that need it, should it be found safe and efficacious in clinical testing. Following IAVI C102, a Phase II trial of the vaccine candidate is also planned in partnership with łÉČËVRĘÓĆľ, with already announced by łÉČËVRĘÓĆľ and the European & Developing Countries Clinical Trials Partnership (EDCTP) for a Phase IIb trial in West Africa.
To better inform the design of future late-stage Lassa vaccine trials, łÉČËVRĘÓĆľ has initiated Enable, the largest-ever Lassa fever research program launched to increase knowledge of the disease burden across West Africa.
The rapid emergence of severe global outbreaks in recent years serves as a stark reminder of the disruptive power of infectious diseases. They can strike at any point and spread around the world â and the potentially deadly Lassa fever is case in point.
While infections are typically reported across West Africa, the increase in global travel enabled the disease to also appear in the U.K. earlier this year. In recognition of its epidemic potential, the world urgently needs a vaccine against this worrisome threat.
IAVI's exciting and important milestone, announced today, is a great step forward in this ambition, gathering critical data from Liberia, a nation already known to be affected by the disease. We look forward to seeing the trial results.
Much of the research and development on IAVI's rVSV platform is performed at the (DDL) in Brooklyn, New York. The DDL is located at the bioscience center in the historic Brooklyn Army Terminal. Since its founding in 2008, the IAVI DDL has become one of the world's leading viral vector vaccine research and development labs, known for innovation and generation of novel vaccine design concepts.
IAVI's LASV vaccine candidate was manufactured by in Leiden, The Netherlands, a contract-development and manufacturing organization focused on delivering sustainable, low-cost manufacturing solutions in the field of infectious disease and cancer. Through its with Batavia, IAVI intends to develop an end-to-end platform for flexible, low-cost production of epidemic preparedness vaccines.
Scientists with IAVI's at Imperial College London validated the key laboratory tests, or assays, needed to measure immune responses in IAVI C102 and conducted technology transfer of these assays to scientists at RH. Additional critical assay work will be conducted by Scripps Research; Tulane University; Viral Hemorrhagic Fever Consortium; The Ragon Institute of MGH, MIT and Harvard; and the Center for Infectious Disease Research.
Results from IAVI C102 are expected in 2023 and will be made available through open-access publications and via scientific meetings to ensure all can benefit from the research.
*RH is part of the National Public Health Institute of Liberia (NPHIL). NPHIL was created in response to the Ebola epidemic in West Africa in 2014-2016. Some of the key goals of the NPHIL include disease surveillance and response and improving epidemic preparedness.
**The Liberian Ministry of Health and the U.S. Department of Health and Human Services (through the National Institute of Allergy and Infectious Diseases) agreed to establish PREVAIL in 2014 to conduct clinical research on Ebola therapeutics and vaccines. PREVAIL has since expanded to address other diseases of public health importance in Liberia.
A local version of this press release is also available to read here.
###
Media Contacts
łÉČËVRĘÓĆľ
[email protected]
+44 7387 055214
IAVI
Rose Catlos
+1 212 847 1049
[email protected]
PREVAIL
Joseph Boye Cooper
T: +231 770.951200
[email protected]
About Lassa fever
Lassa fever is caused by Lassa fever virus, an emerging zoonotic virus that can cause a range of symptoms in humans, including hemorrhage, vomiting, swelling of the face, and pain in the chest, back, and abdomen. Lassa fever virus is most commonly transmitted to humans from an infected rodent known as the multimammate rat (Mastomys natalensis). However, the virus can also spread from person to person via bodily fluids. LASV also has the potential to spread more widely if infected individuals travel and become ill outside the endemic region. The WHO has identified Lassa fever virus as one of the top emerging pathogens likely to cause severe outbreaks in the near future in its .
About the rVSVâG-LASV-GPC vaccine
This vaccine is based on an attenuated, or weakened, strain of vesicular stomatitis virus (VSV) that has been modified to express a Lassa fever virus protein that plays an essential role in establishing viral infection. IAVI the vaccine technology underlying rVSVâG-LASV-GPC from the Public Health Agency of Canada (PHAC). rVSVâG-LASV-GPC is based on an rVSV vector and was developed by scientists at PHAC's National Microbiology Laboratory.
About łÉČËVRĘÓĆľ
łÉČËVRĘÓĆľ is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19, łÉČËVRĘÓĆľ's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus â it has over 20 vaccine candidates against these pathogens in development. łÉČËVRĘÓĆľ has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, łÉČËVRĘÓĆľ initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale and access. These programmes leverage the rapid response platforms developed by łÉČËVRĘÓĆľ's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
łÉČËVRĘÓĆľ's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at .
Follow our for the latest updates. Follow us , , and .
About IAVI
IAVI is a nonprofit scientific research organization dedicated to addressing urgent, unmet global health challenges including HIV, tuberculosis, and emerging infectious diseases. Its mission is to translate scientific discoveries into affordable, globally accessible public health solutions. Read more at .
IAVI initially used rVSV vector technology for HIV vaccine development and now leverages the technology beyond HIV, including for rVSVâG-LASV-GPC development. Funders who have made the development of the rVSV vector possible include the Bill & Melinda Gates Foundation; the Government of Canada; the Danish Ministry of Foreign Affairs; the Government of Japan, in partnership with the World Bank; the Irish Department of Foreign Affairs and Trade; the Netherlands Ministry of Foreign Affairs; the Norwegian Agency for Development Cooperation; the U.K Department for International Development; the U.S. National Institutes of Health (NIH); and through the generous support of the American people from the United States Agency for International Development (USAID).
Follow IAVI on , , , , and , and to our news updates.
About PREVAIL
The Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL) is a joint Liberia-United States biomedical research program internationally recognized for impacting health care delivery. PREVAIL's mission is to conduct collaborative biomedical and public health research in accordance with best practices, to advance science, strengthen health policy and practice, and improve the health of Liberians and people worldwide.