≥…»ÀVR ”∆µ partners, IVI and BBIL, launch global Chikungunya vaccine Phase II/III trial in Costa Rica
Share
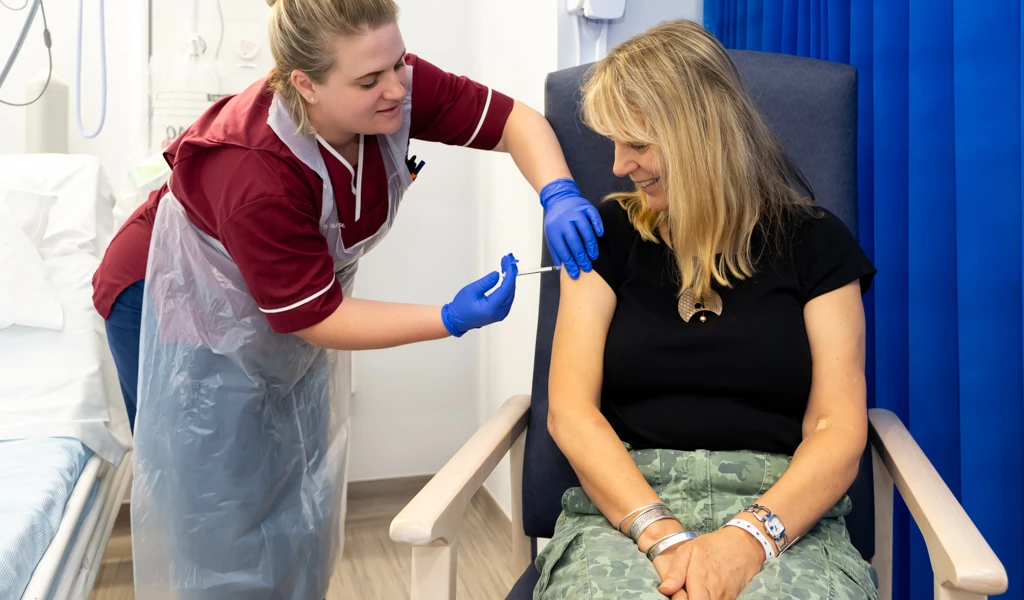
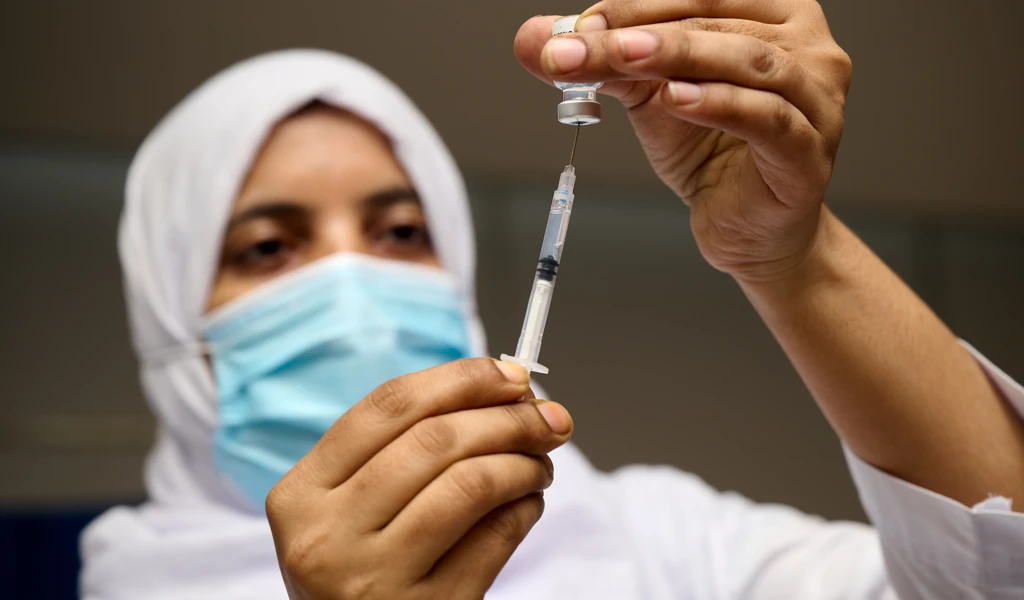
August 24, 2021 ‚Äî SEOUL, Republic of Korea ‚Äî The International Vaccine Institute (IVI) announced today the first participant received Bharat Biotech International Ltd's (BBIL) Chikungunya vaccine candidate (BBV87) in a Phase II/III clinical trial in Costa Rica, marking the start of a multi-country study led by IVI in partnership with BBIL and funded by the Coalition for Epidemic Preparedness Innovations (≥…»ÀVR ”∆µ) with support from the Ind-≥…»ÀVR ”∆µ mission of the Department of Biotechnology, India.
IVI is advancing clinical development of BBV87 through a Phase II/III randomized, controlled trial to evaluate the safety and immunogenicity of a 2-dose regimen of BBV87 Chikungunya vaccine in healthy adults at 9 clinical trial sites across 5 countries with endemic Chikungunya. In addition to the trial at Clinica San Agustin in Costa Rica, trials are expected to begin in Panama and Colombia by September 2021 and in Thailand and Guatemala soon after.
The Global Chikungunya vaccine Clinical Development Program (GCCDP) seeks to develop and manufacture an affordable Chikungunya vaccine with the aim of achieving WHO prequalification to enable its distribution in low- and middle-income countries, consistent with ≥…»ÀVR ”∆µ's core commitment to equitable access, affordability and sustainability. As needed, ≥…»ÀVR ”∆µ or BBIL may propose a third-party for manufacturing of a stockpile of investigational product to be used for further clinical trials in outbreak conditions to advance vaccine development, or pursuant to an emergency use authorization in emergency situations based on national or international guidance (such as by the WHO).
Epidemic preparedness is a vital step in public health care. Bharat Biotech's vaccine candidate is an ingenious, well-researched vaccine, and we thank the first volunteer from Costa Rica for participating in this study. The IVI-led multi-country scale human trial has begun an important trial phase in furthering the evaluation of safety and immunogenicity. As a partner, we are committed towards GCCDP's effort to realize a safe, efficacious vaccine that can help reduce Chikungunya disease burden world over.
As a leader in pandemic vaccines, we have demonstrated unwavering commitment in solving the Chikungunya, Pandemic Flu, Zika and Typhoid problems, as well as our relentless dedication to solve public health challenges through research and development of novel vaccines.
Our Director of R&D, Dr. Sumathy K, Head of the Chikungunya vaccine development program, is actively involved with research and development of this vaccine and works closely with the GCCDP consortium.
We are thrilled to announce that the first participant was dosed with BBIL's Chikungunya vaccine candidate in a multi-country Phase II/III study. The start of this trial in Costa Rica is a significant milestone in the effort to make available a safe, effective, and affordable Chikungunya vaccine for the one billion people around the world at risk of Chikungunya virus infection.
We are grateful to our partner Bharat Biotech, our clinical trial site collaborators and partners, and ≥…»ÀVR ”∆µ for this collective effort that will generate crucial safety and immunogenicity data in regions most affected by Chikungunya.
Today's announcement furthers ≥…»ÀVR ”∆µ's , launched in March 2021, to tackle future epidemics and pandemics, which includes advancing vaccine candidates against known high-risk pathogens such as Chikungunya.
≥…»ÀVR ”∆µ first partnered with IVI and BBIL in June 2020, providing up to US $14.1 million for vaccine manufacturing and clinical development of the BBV87 vaccine candidate. The funding is supported by the European Union's (EU's) Horizon 2020 programme through an existing framework partnership agreement with ≥…»ÀVR ”∆µ. The consortium was also supported with a grant of up to US $2.0 million from the Indian Government's to fund the set-up of GMP manufacturing facilities for the vaccine in India and subsequent manufacture of clinical trial materials.
Chikungunya is a debilitating illness that results in substantial health and economic consequences around the world, including in low- and middle-income countries. Our modern ways of living, globalization, vector adaptation, and climate change could also threaten to advance the spread of the disease further, putting more of the global population at risk.
This Phase II/III study by IVI and BBIL in Costa Rica and other countries in the near future, strengthens efforts to bring an end to this public health crisis. This truly global partnership will provide critical data to inform further clinical development of the vaccine, with the hopes of meeting ≥…»ÀVR ”∆µ's goal to develop, license and gain WHO Prequalification for a Chikungunya vaccine and make it accessible to affected populations.
Chikungunya is a severe and devitalizing disease. Taking into cognizance the need to develop an effective vaccine for chikungunya, the Department of Biotechnology, Government of India has provided financial support under the Ind-≥…»ÀVR ”∆µ Mission, to Bharat Biotech for the Global Chikungunya Vaccine Clinical Development Program (GCCDP).
It is very encouraging to witness the commencement of Phase II/III study of BBV87 in Costa Rica. This milestone is a first step towards developing a promising vaccine candidate against Chikungunya, an exhausting disease.
About BBV87 vaccine candidate
BBIL's BBV87 vaccine is an inactivated whole virion vaccine based on a strain derived from an East, Central, South African (ECSA) genotype. The vaccine has completed standard pre-clinical studies, and an optimum immune response was elicited by the adjuvanted vaccine in phase 1 clinical trials in India. Inactivated virions technology has a safety profile which potentially makes this vaccine accessible to special populations, such as the immunocompromised and pregnant women, that some other technologies cannot reach.
About Chikungunya virus
Chikungunya virus was first identified in Tanzania in 1952, with sporadic outbreaks of the disease reported subsequently across Africa and Asia. In 2004, the disease began to spread quickly, causing large-scale outbreaks around the world. Since the re-emergence of the virus, the total number of cases has been estimated at over 3.4 million in 43 countries.
Chikungunya is spread by the bites of infected female Aedes mosquitoes and causes fever, severe joint pain, muscle pain, headache, nausea, fatigue and rash. Joint pain is often debilitating and can persist for weeks to years.
Climate change could further amplify the threat posed by Chikungunya. As the climate warms, more areas across the world will become habitable for the mosquito vectors that transmit the virus, thereby increasing the size of the human population at risk of infection. For example, in 2007, an outbreak of Chikungunya virus infections was declared for the first time in Europe with more than 200 human cases reported in Italy Since 2014, in the USA, local transmission of the virus has been reported in Florida, Puerto Rico, Texas and the U.S. Virgin Islands.
This press release is also available to read in Spanish and Korean.
###
About the International Vaccine Institute (IVI)
The International Vaccine Institute (IVI) is a nonprofit inter-governmental organization established in 1997 at the initiative of the United Nations Development Programme (UNDP). IVI has 36 countries and the World Health Organization (WHO) on its treaty, including Korea, Sweden, India, and Finland as state funders.
Our mandate is to make vaccines available and accessible for the world's most vulnerable people. We focus on infectious diseases of global health importance such as cholera, typhoid, shigella, salmonella, schistosomiasis, chikungunya, group A strep, Hepatitis A, HPV, TB, HIV, MERS, COVID-19, as well as antimicrobial resistance. For more information, please visit https://www.ivi.int
About Bharat Biotech
Bharat Biotech has established an excellent track record of innovation with more than 145 global patents, a wide product portfolio of more than 16 vaccines, 4 bio-therapeutics, registrations in more than 123 countries, and the World Health Organization (WHO) Pre-qualifications. Located in Genome Valley in Hyderabad, India, a hub for the global biotech industry, Bharat Biotech has built world-class vaccine & bio-therapeutics, research & product development, Bio-Safety Level 3 manufacturing, and vaccine supply and distribution. Having delivered more than 4 billion doses of vaccines worldwide, Bharat Biotech continues to lead innovation, and has developed vaccines for influenza H1N1, Rotavirus, Japanese Encephalitis (JENVAC®), Rabies, Chikungunya, Zika, Cholera, and the world's first tetanus-toxoid conjugated vaccine for Typhoid. Bharat's commitment to global social innovation programs and public-private partnership resulted in introducing path-breaking WHO prequalified vaccines BIOPOLIO®, ROTAVAC®, and Typbar TCV® combating polio, rotavirus, typhoid infections, respectively. The acquisition of Chiron Behring Vaccines has positioned Bharat Biotech as the world's largest rabies vaccine manufacturer with Chirorab® and Indirab®.
To learn more about Bharat Biotech, visit
About ≥…»ÀVR ”∆µ
≥…»ÀVR ”∆µ is an innovative partnership between public, private, philanthropic, and civil organisations, launched at Davos in 2017, to develop vaccines against future epidemics. Prior to COVID-19 ≥…»ÀVR ”∆µ's work focused on developing vaccines against Ebola virus, Lassa virus, Middle East Respiratory Syndrome coronavirus, Nipah virus, Rift Valley Fever virus and Chikungunya virus ‚Äî it has over 20 vaccine candidates against these pathogens in development. ≥…»ÀVR ”∆µ has also invested in new platform technologies for rapid vaccine development against unknown pathogens (Disease X).
During the current pandemic, ≥…»ÀVR ”∆µ initiated multiple programmes to develop vaccines against SARS-CoV-2 and its variants with a focus on speed, scale, and access. These programmes leverage the rapid response platforms developed by ≥…»ÀVR ”∆µ's partners prior to the emergence of COVID-19 as well as new collaborations. The aim is to advance clinical development of a diverse portfolio of safe and effective COVID-19 candidates and to enable fair allocation to these vaccines worldwide through COVAX.
≥…»ÀVR ”∆µ's 5-year plan lays out a $3.5 billion roadmap to compress vaccine development timelines to 100 days, develop a universal vaccine against COVID-19 and other Betacoronaviruses, and create a "library" of vaccine candidates for use against known and unknown pathogens. The plan is available at .
Follow our news page for the latest updates. Follow us on and .
About Ind-≥…»ÀVR ”∆µ
The Department of Biotechnology, Ministry of Science & Technology, Government of India is implementing the Ind-≥…»ÀVR ”∆µ mission ‚ÄòIndia Centric Epidemic Preparedness through Rapid Vaccine Development: Supporting Indian Vaccine Development Aligned with the Global Initiative of the Coalition for Epidemic Preparedness Innovations (≥…»ÀVR ”∆µ)'. Ind-≥…»ÀVR ”∆µ Mission aims to strengthen the development of vaccines for the diseases of epidemic potential in India as well as build coordinated preparedness in the Indian public health system and vaccine industry to address existing and emergent infectious threats in India.
Contact
IVI:
Aerie Em, Global Communications & Media Specialist
+82 2 881 1386 | [email protected]
Bharat Biotech:
Sheela Panicker, enRight PR
+91 98498 09594 | [email protected]
≥…»ÀVR ”∆µ:
Email: [email protected]
Phone: +44 7387 055214